As defined by the FDA, off-label – this is the use of the drug for indications, form, dosage or for a group of people who are not mentioned in the instructions officially approved by the manufacturer. BOTOX® – the world's most famous preparation of botulinum toxin type A, which is used in aesthetic medicine, neurology and urology.
Despite the high clinical efficacy of BOTOX®, many specialists around the world actively practice off-label use of this drug.
And even FDA warnings about the dangers of such practices, as well as many clinical cases of severe complications caused by off-label use of botulinum toxin preparations, do not stop some doctors.
In this article on estet-portal.com, Natalya Sachuk, a dermatovenereologist, cosmetologist, trichologist, dermato-oncologist, head physician of the Farmoza Medical Center, Vinnitsa, and I will tell you why off-label use of botulinum toxin preparations can be dangerous.
Safe and effective: a long way for a drug to be officially registered
At each subsequent stage, the drug is allowed only after it proves its effectiveness and safety in the current experiment.
Initially, the drug is studied at the preclinical stage– in vitro ("in vitro" – outside a living organism) and in vivo (on animals). After that, he can be admitted to the stage of clinical trials, where there are 4 phases of testing.
First phase – the use of the drug in a small (20-80) group of people, the second – in the group of 100-300 participants, the third – several thousand and the fourth – the so-called post-marketing research carried out after its registration.
At each stage, the safety and efficacy of the medicinal product are examined, and all side effects associated with its use are recorded. According to the results of the study, the manufacturer determines for what purposes the use of the drug is successful and safe – this is the basis of the indications prescribed in the official instructions.
Following from this, off-label use of the drug is fraught with the development of severe side effects, and no specialist can guarantee the safety of such an "illegal" drug. its application.
Off-label: not knowing the dosage can be a fatal mistake
The first danger associated with off-label use of BOTOX
® from Allergan – the specialist’s lack of knowledge about the exact required dosage of the drug in such an “ordinary” clinical case. How do experts determine the dosage of the drug if they are not prescribed in the instructions? By trial and error – as a result, at best, botulinum therapy may simply be ineffective. At worst – lead to the development of severe complications.
Botulinum toxin: why poison has no analogues among drugs
Off-label: why they are not in the list of indications of the instruction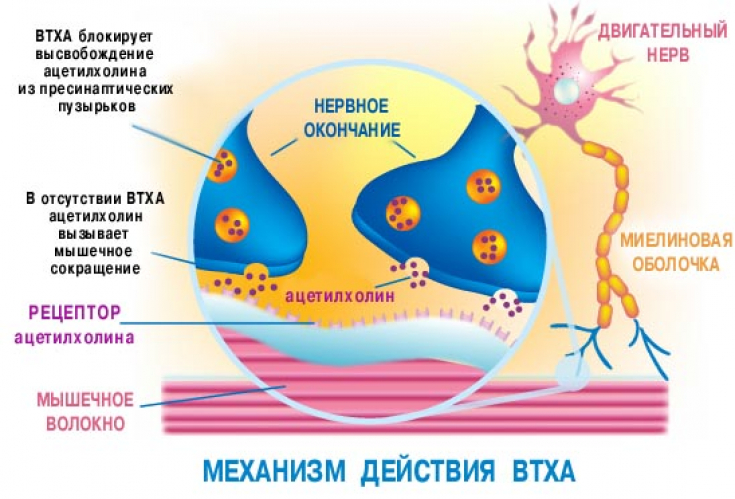
Of course, one cannot but agree that off-label methods of using botulinum toxin can be quite effective and provide a good clinical result.
But before applying the off-label technique, you should ask yourself the question: why didn't the manufacturer include it in the official instructions? After all, it is possible that just such an off-label use of the drug did not pass clinical trials due to its unsafety / inefficiency.
Can we take responsibility that the manufacturer and the FDA have not agreed to take?
Botulinum Therapy – modern solution for primary axillary hyperhidrosis
Official indications for the use of BOTOX preparations®
BOTOX®, which are prescribed in the official instructions.
These include:
1. Neurological disorders: muscle spasms of the hands and wrists in patients after a stroke, tonic blepharospasm, hemifascial spasm, cervical dystonia, deformity of the feet in patients with cerebral palsy (over two years old);2. Urinary disorders: idiopathic overreactivity of the bladder, incontinence;
3. Chronic migraine when other treatments have failed or are contraindicated;
4. Primary axillary hyperhidrosis;
5. Wrinkles in the form of "crow's feet" with a wide smile and glabellar wrinkles with a maximum frown;
6. Lateral wrinkles at the corners of the eyes (wrinkles in the form of "crow's feet") with a wide smile;
7. Vertical wrinkles between the eyebrows in patients under the age of 65.
Thus, the estet-portal.com team urges all specialists who use off-label botulinum therapy methods in their practice to always consider the potential risks associated with such practice and value the safety and trust of their patients.
Original drugs of the company "Allergan" You can only purchase from an authorized distributor.
World-class research: higher doses of BOTOX®







Add a comment