Neurologist Andrey Korolev tells about botulinum therapy for post-stroke spastic paresis.
Spastic paresis
Spastic paresis that develops after a cerebral stroke, as a rule, persists throughout the patient's life. At the same time, secondary changes in muscles, tendons and joints develop. In this regard, it is relevant to develop approaches to the therapeutic effect on muscle spasticity, including the use of botulinum toxin injections.

neurologist, candidate of medical sciences, member of the Association of Neurologists of St. Petersburg, Interregional Public Organization of Botulinum Therapy Specialists
Spastic muscular hypertonicity is characterized by the appearance of increasing resistance during the first rapid passive movements and then a sudden decrease in it - the phenomenon of "jackknife" according to Sherrington. Currently, spasticity is understood as a motor disorder that is part of the upper motor neuron lesion syndrome, characterized by a speed-dependent increase in muscle tone and accompanied by an increase in tendon reflexes as a result of hyperexcitability of stretch receptors.
Once developed, spastic paresis, as a rule, persists throughout the life of the patient. Moreover, if spasticity persists for a long time, secondary changes develop in muscles, tendons and joints (fibrosis, atrophy, contracture). The treatment of spastic paresis is still a practically insoluble task. In this regard, the development of approaches to the therapeutic effect on muscle spasticity, based on the study of the mechanisms of its development, is so relevant.
To correct muscle hypertonicity, various methods of physiotherapy, physiotherapy, reflexology, as well as pharmacological treatment in the form of prescribing muscle relaxants are often used.
Botulinum neurotoxin type A preparations as an effective treatment for post-stroke static.
In recent years, botulinum neurotoxin type A preparations have been used in the treatment of post-stroke spasticity. This method of treatment has been proposed for mastering by practicing physicians relatively recently, despite the fact that the first scientific report on the use of botulinum toxin blockades appeared in the press more than 20 years ago.
In comparison with the existing methods of treating muscle hypertonicity, the local administration of botulinum toxin has a number of undoubted advantages. First, the treatment is well tolerated and is not associated with a risk of serious complications. Secondly, it is possible to select one or more muscles for injection and select the dose of the drug that provides the desired degree of relaxation.
Mechanism of action of botulinum toxin
Botulism is known to be a severe infectious disease manifested by peripheral muscle paralysis and autonomic disorders due to impaired peripheral cholinergic mediation caused by botulinum neurotoxin. Currently, 8 serological subtypes of botulinum toxin are known: A, B, C1, C2, D, E, F, G. Botulism in humans can be caused by serotypes A, B, E, F, G, but type A is the most potent.
Both in its natural form and as a drug, botulinum toxin is a mixture of different proteins. Their main components are neurotoxin and non-toxic proteins. The neurotoxin consists of two polypeptide chains (light - 50 kDa and heavy - 100 kDa) connected by one disulfide group and one zinc atom. Such a structure of the neurotoxin molecule causes the lability of its conformation and instability to the action of mechanical, physical and chemical factors leading to the loss of biological activity. In their dosage form, neurotoxin chains are surrounded and stabilized by large peptide molecules of hemagglutinins and non-toxic non-hemagglutinin proteins. The large molecular weight of the non-toxic part of the complex (730 kDa) prevents both the breakdown of the neurotoxin, and its rapid diffusion into the surrounding tissues, thereby providing localized exposure. However, the presence of protein molecules is a factor contributing to the formation of neutralizing antibodies to the entire neurotoxin-hemagglutinin complex, which may cause secondary insensitivity of patients to repeated injections of the drug.
The amino acid composition of neurotoxin type A has been deciphered, the light chain contains 448 amino acids, and the heavy chain contains 848. Other serotypes of botulinum toxin differ in the number and sequence of amino acids, mainly in the light chain.
The principal mechanism of action of all types of botulinum toxins is the presynaptic blockade of the release of acetylcholine from the nerve terminal and the peripheral cholinergic synapse.
The transmission of a nerve impulse in a cholinergic synapse occurs in several stages. In the presynaptic nerve terminal, acetylcholine is constantly synthesized and accumulated in the form of vesicles that are transported to the presynaptic membrane so that the mediator molecule can enter the synaptic cleft and bind to specific cholinergic receptors on the postsynaptic membrane. At this site of the postsynaptic membrane, a membrane potential arises and a contraction of the muscle fiber occurs. However, the process of transport of acetylcholine vesicles to the presynaptic membrane does not occur spontaneously, but actively with the help of a complex of special transport proteins, the main of which are SNAP-25, syntaxin and synaptobrevin.
It is transport proteins that are the target of botulinum neurotoxins. When botulinum toxin enters a muscle or other target organ (with blood flow in case of botulism or for therapeutic purposes when injected), molecules of the toxin complex reach the nerve terminals of axons, attach to them, and then the neurotoxin part is introduced into the cytosol of the nerve terminal, where it breaks up into a short and long chain. The short chain (which is a zinc-dependent protease) irreversibly and specifically cleaves the transport protein, thereby preventing the release of acetylcholine into the synaptic cleft and muscle contraction.
Botulinum toxin injection effects and how to prolong them
With intramuscular injection of botulinum toxin, two effects develop: direct inhibition of alpha motor neurons at the level of the neuromuscular junction and inhibition of the gamma motor neuron cholinergic synapse at the intrafusal fiber. A decrease in gamma activity leads to relaxation of the intrafusal fibers of the muscle spindle and reduces the activity of 1a afferents. This leads to a decrease in the activity of both muscle stretch receptors and the efferent activity of alpha and gamma motor neurons. Clinically, this manifests itself in a pronounced relaxation of the injected muscles and a significant reduction in pain in them.
When administered locally at therapeutic doses, botulinum toxin does not cross the blood-brain barrier and does not cause significant systemic effects. The process of presynaptic cleavage of transport proteins by botulinum toxin is irreversible and takes an average of 30–60 minutes; therefore, specific botulinum antitoxin is effective only within half an hour after the toxin reaches the target organs. Despite the fact that the cellular effects develop very quickly and irreversibly, the clinical muscle relaxant effect of the drug after the injection begins to appear after a few days. However, there are observations of both an immediate onset of the effect and a delayed effect of 3-4 weeks.
After 1–2 months after the injection, the process of regrowth of new nerve terminals from axons, where acetylcholine transport was previously blocked, begins, with the formation of new functionally active neuromuscular synapses (sprouting), which ultimately leads to the restoration of muscle contractions after 3 -6 months after injection, but sometimes the effect lasts up to 1 year or more.
Histological studies have shown that even after 30 repeated injections into the same muscle, irreversible de-innervation and atrophy do not occur.
It is extremely important to develop ways to enhance and prolong the effect of the injection, since frequent repeated injections can lead to the formation of antibodies, and also significantly increase the cost of treatment. It was found that more persistent and complete effects of the toxin are manifested with maximum muscle contraction, with a sufficient intracellular concentration of calcium ions (possibly also potassium), when exposed to low temperatures. Therefore, during preparation for a therapeutic injection, the patient is recommended to prescribe calcium and potassium preparations with vitamin D 2 weeks in advance, immediately before the injection and immediately after it - to cool the injection area, and also to intensively strain the injected muscles for 15–30 minutes after the procedure.
The size of the field of denervation caused by the injection of a toxin depends on the dose of the toxin and the volume of the injected solution. The best results are achieved when the drug is evenly distributed at several points along the same muscle. In addition, a more complete blockade of the nerve terminals occurs when the toxin is injected near the motor end plates of the peripheral nerve.
Evidence has now accumulated that the effects of botulinum toxin are much more complex and broader than the temporary local block of conduction in the terminals of alpha motor neurons. Apparently, there is a blockade of both intrafusal and extrafusal fibers, which can explain the wider area of action of the toxin on the terminals of sensitive fibers of various modalities. In particular, this may explain the rapid analgesic effect of botulinum toxin injection. Due to the mechanism of deafferentation of muscle spindle receptors and other types of sensitive systems, botulinum toxin can have indirect effects on the overlying parts of the central nervous system. When studying the motor potential, a decrease in the latent periods of its components was revealed,
Method of prescribing botulinum toxin preparationsPrescribing more than 300-400 IU during a single injection session should be avoided. To date, no serious side effects of botulinum toxin preparations have been identified when used at recommended doses. Excessive muscle weakness may occur, but muscle strength is restored over time. Secondary resistance to the drug may also be observed, for the prevention of which it is recommended to make an interval between injection sessions of at least 12 weeks. There was also no interaction effect of botulinum toxin and centrally acting oral muscle relaxants.
Today, the widespread use of botulinum therapy for post-stroke spasticity is largely limited by the high cost of the drug. However, a study conducted in Germany on the effectiveness of three treatment options for post-stroke spasticity: physiotherapy, botulinum therapy + physiotherapy, baclofen + physiotherapy - showed that the reduction in spasticity with the combination of botulinum toxin and physiotherapy was three times more significant than with the use of baclofen and physiotherapy, and ten times times more than with physiotherapy alone. The cost/effectiveness estimate for the treatment of post-stroke spasticity was lower with botulinum toxin and physical therapy than with other treatments.
For a more informed prescribing, O'Brien C. (Muscle and Nerve, 1997) recommends the following algorithm for prescribing botulinum toxin drugs for post-stroke spasticity (Figure below).
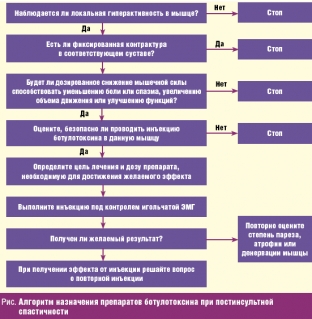
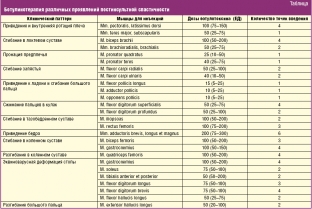
In the lower extremity, due to the increase in muscle tone in the posterior leg muscle group, it becomes clear which of the muscles of the posterior leg group (calf or soleus) plays a leading role in spasticity. If a high tone in the ankle joint (during dorsiflexion of the foot) is determined with an extended knee joint, but significantly weakens with flexion, then this indicates the leading role of the gastrocnemius muscle in spasticity, since when the knee is flexed, the gastrocnemius muscle relaxes, which is involved both in knee flexion, and in plantar flexion of the ankle joint. In these cases, 100–150 units of botulinum toxin are injected into four points of the gastrocnemius muscle (25–35 units at each point). In cases where spasticity is equally caused by both gastrocnemius and soleus, additionally, at a dose of 50–100 IU in two points (25–50 IU each), the drug is injected into the soleus muscle. The choice of other target muscles of the leg also depends on the clinical pattern [34].
The maximum effect after botulinum therapy is observed, on average, 2-3 weeks after the injection. The recurrence of muscle spasticity usually occurs 4-6 months after the injection of botulinum toxin, which requires a repeat of the procedure.
In conclusion, it should be noted that in all cases, after botulinum therapy, active physiotherapy and physical rehabilitation are required. Botulinum therapy does not replace physiotherapy and exercise therapy, which is the basis of the rehabilitation program for patients with stroke, but is only an integral part of a comprehensive rehabilitation treatment aimed at improving motor functions.
A significant number of issues related to the use of botulinum toxin in spasticity require further study. First, is it safe and effective to use large doses of botulinum toxin, which is often necessary for severe spasticity in many muscles in patients after a cerebral stroke? Secondly, how long after the stroke should botulinum therapy be performed? Thirdly, what are the optimal doses and points of injection of the drug into various muscles? Fourth, what combination of medical rehabilitation and botulinum toxin therapy is optimal for different periods of prescription of the disease?
Thus, the problem of restorative treatment of post-stroke spastic muscle hypertonicity using botulinum toxin has been one of the important places in practical neurology and neurorehabilitation for many years and requires further study.
According to lvrach.ru






Add a comment