Type 2 diabetes mellitus (DM2) is a disorder of carbohydrate metabolism caused by predominant insulin resistance and relative insulin deficiency.
The number of people with undiagnosed pathology actually exceeds the number of identified patients by 3-4 times.
According to world statistics, every 13-15 years the number of people with diabetes is doubling.
The above facts show a significant prevalence of type 2 diabetes in the world.
Therefore, everything related to this disease is relevant, especially with regard to treatment.
Find out in the article on estet-portal.com about the modern possibilities of drug therapy for diabetes mellitus 2 type.
- Glucagon-like peptide-1 receptor agonists in the treatment of type 2 diabetes mellitus
- Efficacy and safety of Ribelsus for the treatment of type 2 diabetes
- Recommendations for the use of Ribelsus for the treatment of type 2 diabetes Glucagon-like peptide-1 receptor agonists in the treatment of type 2 diabetes This year, the US Food and Drug Administration (FDA) approved a new drug for the treatment of type 2 diabetes. The drug, created by pharmaceutical company Novo Nordisk, is called Rybelsus (Rybelsus, semaglutide), an oral glucagon-like peptide-1 (aGLP-1) receptor agonist
Follow us on
Instagram!
Ribelsus was the first drug in this group in the world sold for oral administration, the rest of the drugs in this group are injectable. It is known that previously drugs based on peptide compounds were not used orally due to the destruction of the aggressive environment of the stomach and duodenum. The pharmacological feature of Ribelsus, which allows it to be absorbed in the gastrointestinal tract, is that this drug is combined. This facilitates the absorption of semaglutide in the stomach by a local increase in
pH
which leads toincreased solubility of the drug
andprotects it from proteolysis.
Thus, oral administration of the peptide preparation becomes possible. The combination of and sodium caprylate creates a kind of microenvironment inside the gastric mucosa, which allows the drug to be absorbed and realize its effects no worse than injectable GLP-1 solutions
ia.
Sirtuins –longevity proteins for skin and body rejuvenation Efficacy and safety of Ribelsus for the treatment of type 2 diabetes
The PIONEER large-scale clinical trial included 10 phase III clinical trials, in which a total of 9.5 thousand patients took part, which confirmed thesafety and effectiveness of Ribelsus.
The drug was evaluated both in monotherapy and in combination with metformin, sulfonylurea drugs, sodium-glucose cotransporter type 2 (SGLT2) inhibitors, and insulin.The drug has also been tested in patients with type 2 diabetes mellitus with moderate renal insufficiency (glomerular filtration rate 30-59 ml min / 1.73 m2).
Riebelsus result did not depend on:
age;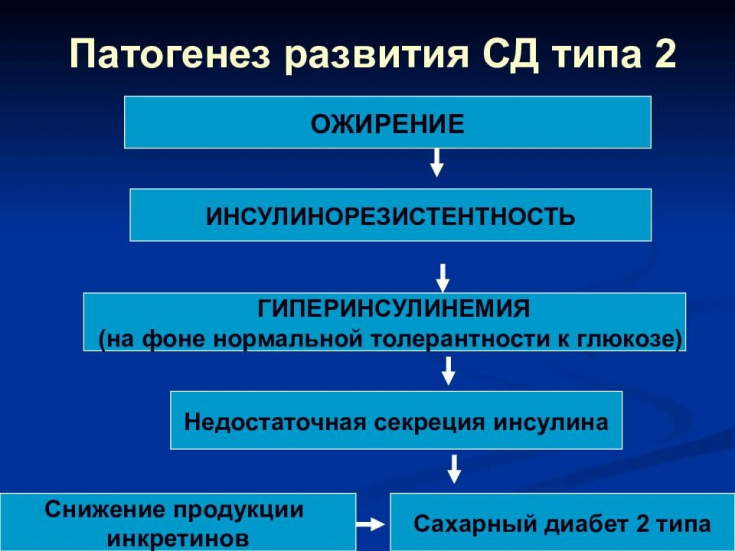
race; ethnicity;
- body mass index;
- duration of type 2 diabetes;
- degrees of impaired renal function.
- Most clinical trials in the semaglutide groups showed a statistically significant advantage
- over the control groups in reducing the level of glycated hemoglobin (HbA1
c) and body weights: by 1.0-1.8% and 2-5 kg respectively. Also demonstrated a reduction in the risk of 21% of cardiovascular adverse events: myocardial infarction or stroketa.
Drug therapy for diabetic complications Recommendations for the use of Ribelsus in the treatment of type 2 diabetes
Ribelsus will be available in blister packs of 7 and 14 mg tablets.It is noted that when taking this drug, do not split, split or crush the tablet to avoid deterioration of absorption in the gastrointestinal tract. The medicine is taken on an empty stomach
andwith a small amount of water
, which is necessary for sufficient absorption in the stomach, and not the passage of the drug in transit into the duodenum.Patients can start taking food, other liquids and drugs no earlier than 30-60 minutes after taking the drug.
This is to ensure that the microenvironment created allows the drug to be absorbed and enter the systemic circulation.
There are also
restrictions on taking the drug.
type 1 diabetes and
and
The drug has not been studied in patients under 18 years of age
, andis not a first-line drug for the treatment of diabetes. The drug is contraindicated in patients with an individual or family history of medullary thyroid cancer
, as well as inmultiple endocrine neoplasia type 2 syndrome. It is not known how undesirable the use of the drug in the presence of pancreatitis, during pregnancy and lactation.







Add a comment