LaViv (Azficel-T) – a relatively new dermal filler, made on the basis of autologous (own) fibroblasts of the patient, which is intended for the correction of nasolabial folds. This autologous filler is the only personalized cell therapy of its kind approved by the FDA for use in aesthetic medicine on June 22, 2011. The developer of LaViv is the American company Fibrocell Science, the production of fillers is made in the laboratories of the company.
Manufacturing process, indications and course of injections of LaViv autologous filler
The product uses a patented technology: first, the patient's own cells (fibroblasts) are taken from the earlobe, then they are sent to the Fibrocell laboratory, where they are isolated and cultured for about 3 months, after which they are frozen and stored until use.
As is known, fibroblasts are responsible for the synthesis of extracellular matrix proteins, including collagen, which form the structural frame of the skin.
Indications for LaViv are moderate to severe nasolabial folds in adult patients.
The course of injections of autologous fibroblasts into the nasolabial zone consists of three procedures with an interval of 3-6 weeks. The most pronounced results are observed after the last session, the effect lasts for at least 6 months.
Azficel-T technology, based on the introduction of an autologous filler based on the patient's own fibroblasts, ensures the physiology of the rejuvenation procedure. Natural restoration of cells that died as a result of age-related processes in the body.
Before and after the LaViv autologous filler procedure:
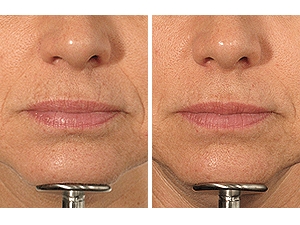
Before injections After injections
Fibrocell announced that dermatologists and plastic surgeons who have received special training and certification from the company will be allowed to conduct the procedures. The training program will include:
- biopsy taking;
- preparation and transport of the patient's cell material;
- preparing the patient for the procedure;
- introduction technique;
- logistics control to ensure that the patient is injected with his own cells.
Clinical studies with LaViv have demonstrated good patient tolerance. Adverse reactions of moderate severity are associated with the injection method of administration and resolve within 1 week after the procedure.







Add a comment