The need to optimize the development of influenza vaccines that can provide powerful protection against broad spectrum strains and subtypes remains relevant.
Toward this goal, identification of new neutralizing epitopes that are the target of protective antibodies is helping.
Influenza vaccine development has focused on hemagglutinin.
However, recently, another surface antigen has been taken up as a potential target in the development of a universal vaccine.
Find out about the results of the study in the article on estet-portal.com.
- Approaches to developing a universal influenza vaccine
- Immune response to influenza infection
- Clinical implications of developing a universal influenza vaccinea
Approaches to developing a universal influenza vaccine
A new study presented by scientists at The Scripps Research Institute, USA, in collaboration with researchers at the Washington University School of Medicine in St. Louis, describes three human monoclonal antibodies isolated from H3N2 -infected donor.
Follow us on Instagram!
According to the results of the study, the ability of these antibodies to bind to neuraminidase of various subtypes of influenza A and B viruses was revealed.
Moreover, the ability of monoclonal antibodies to neutralize the influenza virus, to serve as mediators of effector immune functions, as well as the ability to inhibit neuraminidase activity by directly binding to the active site has been proven.
Why actinomycotic inflammation of the salivary glands develops
According to the researchers, further detailed description of the structural and functional properties will contribute to the successful development of universal vaccines against the influenza virus based on the tropism for neuraminidasee.
These results provide evidence of a new approach in dealing with complex influenza cases, including pandemics.
Immune response to influenza infection
Explaining the specifics of the research topic, the authors, first of all, focused on the properties of the protein located on the surface of viral particles.
This protein causes the ability of infected host cells to release the virus for further spread.
Neuraminidase inhibitors currently available in the group, in particular oseltamivir, act by acting directly on the specified viral enzyme.
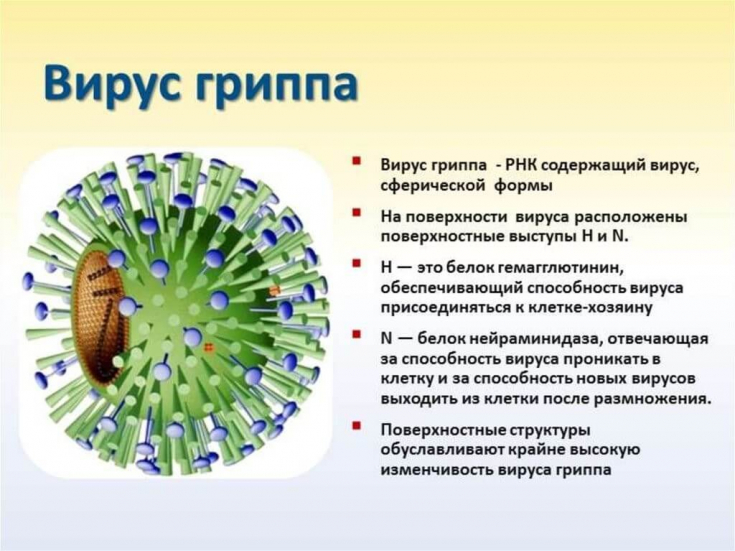
At the same time, the variant series of neuraminidase is characterized by significant diversity, determining the viral strain.
Therefore, these medicines are not always effective, especially given the development of resistance.
In addition, this results in annual need to develop and produce new vaccines that will match the most relevant and common circulating viral strains.
Note to travelers: Ebola virus
In a new study, when studying a blood sample from a patient hospitalized for influenza, scientists noticed unusual differences.
So, besides the content of antibodies against hemagglutinin − the main surface protein of the influenza virus, the presence of other antibodies with an unknown target of action was determined.
After screening the detected antibodies for complementarity of their structure in the known spectrum of neuraminidase proteins, it was found that at least one of the three antibodies found blocked the activity ofall known types of neuraminidase influenza viruses .
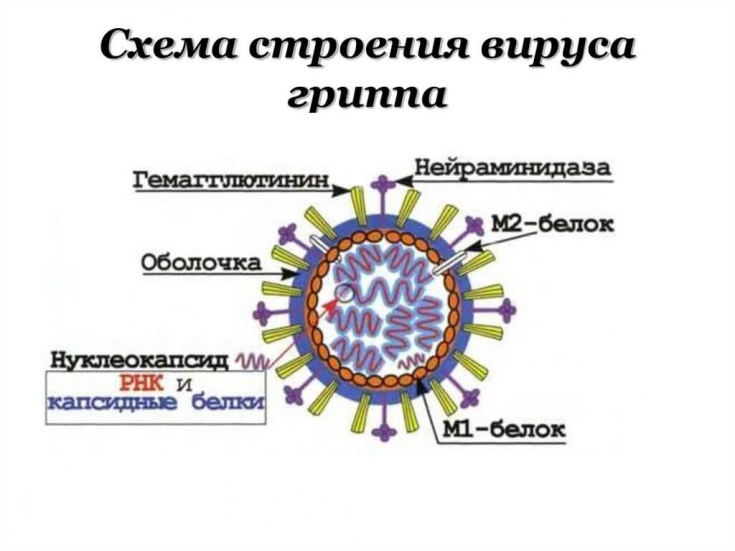
Clinical implications of developing a universal influenza vaccine
Neuraminidase inhibitorscurrently known are recommended to be used within the first 24 hours from the onset of the first symptoms of the disease.
However, the development of a new drug that can be effectively used even at a later date from the onset of the disease would require a clear understanding of the direct mechanism of its action on neuraminidase.
For this purpose, antibodies structure mapping at the moment of their binding to neuraminidase was carried out.
It was established that each of the antibodies formed a special helix, which sequentially moved along the active center of neuraminidase, like a stick along a gear wheel structure.
What threatens a latent herpetic infection
Thus, the helical structures prevented the release of new viral particles by neuraminidase from the cell surface, thereby disrupting the process of viral replication in the cells of the host organism.
Therefore, inactivation of neuraminidase was achieved without direct contact with the variable sites of the enzyme, blocking the conserved active site locus and thus achieving a large spectrum of antiviral action.
This neuraminidase site is virtually unchanged among distantly related strains of the influenza virus.
This is due to its biological role, because even minimal changes in its morphology can block the activity of the viral protein, inhibiting the ability of the virus to reproduce.
Effective prevention of herpes zoster
Summarizing the results obtained, the authors of the presented work noted that a clear understanding of the mechanism of action of universal antibodies to neuraminidase opens up an alternative way to develop new vaccines against the influenza virus, the use of which would contribute to the synthesis of such antibodies in the human body.
Therefore, at this stage, researchers are working to develop a treatment and vaccine against influenza virus based on antibodies 1G01.







Add a comment