An effective treatment for Ebola infection has not yet been formally proposed.
Existing measures constitute intensive symptomatic therapy:
- hemodynamic support;
- electrolyte balance correction;
- blood transfusion;
- elimination of coagulopathy;
- therapy aimed at combating secondary infection.
Therefore, the issue of preventing this disease is very acute.
Find out in the article on estet-portal.com about the features of the clinical picture and the possibility of preventing infection Ebola virus.
- Clinical presentation of Ebola virus infection
- Ebola virus epidemiology
- Prospects and development of an Ebol vaccinea
Clinical presentation of Ebola infection
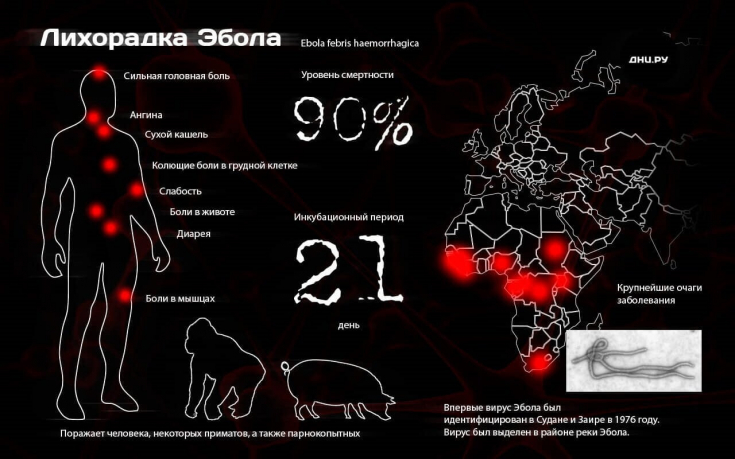
The incubation period is between 2 and 21 days.
Illness starts suddenly:
- an intense headache appears, mainly in the frontal or occipital areas;
- body temperature rises to 39-40 ° C;
- expressed dryness in the throat worries.
Follow us on Instagram!
abdominal pain, diarrhea, vomiting appear. In
severe forms, on the 4th-5th day of the disease, maculopapular (morbilliform) rashes develop, more intense on the lower half of the trunk and extensor surface of the extremities, an injection of the conjunctiva is also observed. These phenomena are accompanied by:
bleeding from gums, nose;
- blood in feces and vomit;
- diarrhea causing severe dehydration;
- dry mouth that interferes with food and water intake.
- Death occurs
What threatens a latent herpetic infection Ebola epidemiology
− common name for 6 species of the genus Ebolavirus of the filovirus family: Zaire ebolavirus; Sudan ebolavirus; Reston ebolavirus; Tai Forest ebolavirus; Bundibugyo ebolavirus; Bombali ebolavirus.
The mortality rate of patientswho contracted Ebola virus has been in the range of 25-90% in past outbreaks. The largest outbreak to date occurred in West Africa in 2014-2016. with a fatal outcome of more than 11 thousand people.
Current outbreaks in the Democratic Republic of the Congo (DRC) caused by
Ebolovirus Zaire (Zaire ebolavirus) have resulted in approximately 67% fatal cases.
Affectionate killer: why hepatitis C is dangerous The European Medicines Agency − EMA has decided to grant approval to the American
pharmaceutical companyMerck & Co. to promote their vaccine to the market, which allows the distribution of this vaccine, especially in Africake. Prospects and development of an Ebola vaccine
VaccineMerck & Co. was first patented in 2003 when it was used on an emergency basis to reduce the spread of the Ebola virus in the DRC, which has killed about 2,000 people since the beginning of last year . The vaccine was also used during another outbreak in 2018 in the DRC and in 2015 − in Guinea.
Beware of infectious mononucleosis in children The vaccine has already been used to protect more than 250,000 people in the DRC and could be classified as an
emergency during the Ebola outbreak. The European organization is supporting the manufacture and delivery of Ebola vaccines and is looking forward to a global supply that could work quickly in future outbreaks.
VaccineMerck & Co., which is sold under the name Ervebo (Ervebo) and is known to scientists as rVSV-ZEBOV-GP, tested in clinical trial in Guinea and at the end of the 2014-2016 Ebola outbreak in West Africa. The vaccine initiates an immune response in the form of the production of neutralizing antibodies against the Ebola virus, which circulate in the body for a long time.
Ervebo vaccine has been tested in approximately 16,000 clinical cases of Ebola infection.
These tests
proven the effectiveness of Ervebo. If one adheres to the so-called
ring vaccination(when immunization covers all persons who have been in contact with the patient, as well as those who have been in contact with them), the vaccine provides protection at almost the maximum level - 97.5% ( 95% confidence interval (CI) 95.8-98.5). In its time, ring vaccination helped eradicate smallpox completely.

, after Yervebo's approval, put her through the pre-qualification of medicines, confirming the quality, efficacy and safety of the vaccine. This means that Yervebo can safely be bought by various UN agencies and GAVI with subsequent distribution among countries with an active spread of the Ebola virus.
Rationale for prescribing probiotics for gastroenteritis Further, it is important to determine whether the vaccine can be used not only during epidemics, but, for example,
as a prophylactic agentfor healthcare workers who may be exposed to the Ebola virus in the distant future. To do this, it is necessary to find out how long the effect of the vaccine lasts, and whether an additional dose can continue the immunity.
Such studies are being conducted on
rVSV-ZEBOV-GPand alternative vaccines, the results of which will be available shortly.







Add a comment