How to choose a safe preparation for contouring , or “soft tissue augmentation” in modern aesthetic medicine, this is how the scientific medical literature defines a set of injection techniques aimed at restoring or increasing the volume of facial tissues, wrinkle removal, etc.?
Especially for the readers of estet-portal.com about which category of medical devices preparations for soft tissue augmentation belong to, what regulatory documents regulate this and who should monitor the safety and quality of these products, said the surgeon, dermatologist, specialist in aesthetic medicine Vladimir Okhten.
- What category of medical devices are contouring preparations
- Who controls the quality of medical devices
- How a medical device is put into service
- How in practice the doctor can determine the safety of the drug used
- Why are all these points so important to the safety of the enemy?cha
What category of medical devices do contouring drugs belong to
V.O.: Preparations for contour plastics are medical devices. For their more specific identification, there is Global Medical Device Nomenclature (GMDN; Global Medical Device Nomenclature), which is a list of generic (generic) names used to identify all products classified as medical devices.
In 2019, the Ministry of Economic Development and Trade of Ukraine, by order No. 159 of February 5, 2019, approved the national classifier NKMI 024: 2019 "Classifier of medical devices", and it is harmonized with the international nomenclature of medical devices Global Medical Device Nomenclature (GMDN) , 2018.
Principles of building a personal brand in the aesthetic medicine market
According to this nomenclature, soft tissue augmentation products are classified as Dermal tissue reconstructive material and further depending on the material (bacterial, animalwow, human, synthetic origin) and the presence of an anesthetic.
Who controls the quality of medical devices
V.O.: Control over the safety and quality of medical devices in the world is carried out by certain structures, the most famous of which are:
- Food and Drug Administration, FDA, US FDA, Medicines and Healthcare products Regulatory Agency, MHRA
- United Kingdom.
of 06/14/1993 93/42/EEC (Medical Devices Directive – MD Directive, MDD), which is replaced by the Medical Device Regulation as of May 25, 2017 -MDR (Manufacturers of approved medical devices currently have a three-year transition period until May 26, 2020.
Education of doctors: where to get quality knowledge
from July 1, 2015, Technical regulations in the field of medical devices developed on the basis of the relevant Directives of the European Union. became mandatory for use In particular, the circulation and use of injectable implants in Ukraine is allowed if they are confirmed to comply with the medical technical regulations approved by the Resolution of the Cabinet of Ministers of Ukraine No. 754 dated 02.10.2013.
According to this regulatory document, namely Annex 2, soft tissue augmentation products are classified as implantable medical devices and depending onthe degree of potential risk of use
in accordance with the provisions of Directive 93/42 / EC of 14 June 1993 "Medical devices" and DSTU 4388: 2005 "Medical devices. Classifications depending on the potential risk of use. General requirements" refer to class III. How the medical device is put into service
V.O.:A mandatory requirement of technical regulations regarding medical devices is the appointment of
Authorized representative of the manufacturerin Ukraine, if the manufacturer is not a resident of Ukraine, and this is almost all drugs available on the market. In order to put medical devices into circulation and operation on the territory of Ukraine, it is necessary to undergo a conformity assessment procedure for these devices and apply a national mark of conformity.
Read the most interesting articles inTelegram!

na for Medicines and Drug Control
http ://dls.gov.ua. How can a doctor determine the safety of the drug used in practice
VO:How does it look in practice, what information about the drug for safe use should the doctor know and be able to check? We can consider this on the example of such a large and well-known manufacturer as the Swiss company
TEOXANE Laboratories. Manufacturer:
THEOXANE S.A., 105 Rue de Lyon, Geneva, Switzerland;- Authorized representative of the manufacturer in Ukraine: Ledzhi Group LLC, 01103, Kiev, Druzhby Narodov boulevard, 18/7;
- National mark of conformity (approved by the Resolution of the Cabinet of Ministers of December 30, 2015 No. 1184 "On approval of the form, description of the mark of compliance with technical regulations, rules and conditions for its application"):

- Certificate: No. PR/692-19 posted on the official website of LLC "Ukrainian Scientific Institute of Certification"
- https://uni-cert.ua/
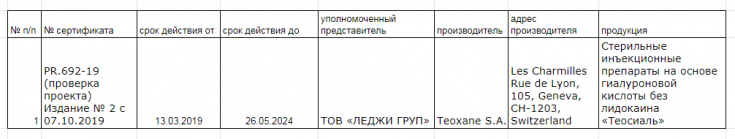 It should be noted that now in Ukraine there is no unified register of certified medical devices, all information about certified products is located on the official websites of each conformity assessment body separately
It should be noted that now in Ukraine there is no unified register of certified medical devices, all information about certified products is located on the official websites of each conformity assessment body separately
Outer packaging and instructions: must be in Ukrainian and contain the information specified in paragraph 1, paragraph 2, paragraph 3, paragraph 4.
- Thus, we can see that products of
TEOXANE comply with all regulatory documents for its safe use. Why are all these points so important for the safety of the doctor
V.O.:Today, according to
requirements of the current legislation, the authorized representative of the manufacturer has a number of obligations, one of which stipulates that he must immediately inform the manufacturer about complaints and reports from medical professionals, patients about suspicious incidents related to a medical device, because only the manufacturer can decide on further corrective actions or even product recalls. State market supervision and control is carried out by the "State Service of Ukraine for Medicines and Drugs Control" by conducting scheduled and unscheduled inspections of product characteristics.
Beauty with a bright future: the secrets of perfect skin
Choosing a quality and proven product – You are in the legal field and guarantee the quality and safety of contouring to your patient.
More useful and interesting information on our channel on
YouTube:







Add a comment