Today, antihistamines (AHP) are one of the basic means of treating a wide range of allergic and inflammatory diseases and are actively used by doctors in clinical practice. The main indications for the appointment of AGP are allergic rhinoconjunctivitis, various types of urticaria. These drugs are also actively used in dermatology to reduce the intensity of itching in dermatoses (atopic dermatitis, pruritus, eczema), as well as to stop acute allergic reactions to food and drugs, insect bites and stings.
The history of histamine control drugs
The history of the creation of these drugs began in 1910, when histamine was discovered. Histamine – a physiological regulator of tissue and metabolic homeostasis and one of the most studied molecules in medicine. The main depots of histamine in tissues are mast cells, in the blood – basophils.
Histamine is involved in the interaction between cytokines and inflammatory cells, promotes cell migration to the area of inflammation.
Histamine is involved in a complex bidirectional interaction between cytokines and inflammatory cells or their precursors, promotes cell migration to the area of inflammation, stimulates lymphocytic activity, regulates the functioning of eosinophils, neutrophils and mast cells, and is directly involved in the generation of major allergic symptoms, such as the common cold, sneezing, nasal congestion, pruritus, urticaria. It is a key mediator in all clinical symptoms of allergy, activating cell surface specific receptors.
May be interesting Allergy to cosmetics and how to deal with it
4 types of histamine receptors are currently known
H1 receptors are expressed on many cell types, including mast cells, basophils, dendritic cells, endothelial cells, and smooth muscle cells.
They play an important role in initiating the pro-inflammatory activity of immune cells when they interact with histamine and thus determine the clinical manifestations of an allergic reaction. The main goal of suppressive therapy with H1 blockers is competitive inhibition of histamine H1 receptors to prevent the development of histamine-initiated effects in allergic rhinitis (AR), urticaria and other allergic processes.
Recent studies have shown that normally H1 receptors are present in cells in two states: active and inactive.
Histamine binds to active receptors and shifts the dynamic balance in their direction. Antihistamines stabilize the H1 receptor in an inactive state, being their inverse agonist. Thus, antihistamines prevent or minimize histamine-induced inflammatory responses.
AHPs have been widely used in clinical practice for over 70 years. The first AGPs were developed in 1937 by French scientists A. Staub and D. Bouvet, who worked at the Pasteur Institute in Paris (France). However, due to the high toxicity, the use of these compounds turned out to be impossible.
In 1942, the famous French scientist H. Halpern introduced into clinical practice phenbenzamine, and then pyrilamine, related to the first generation of AHD. Subsequently, many drugs of this group were developed and introduced into clinical practice. In the early 1980s A new generation of AGPs have been developed. The main difference between these 2 groups of drugs is the presence or absence of a sedative effect. H1-AHP of the 1st generation (sedative) is called classical, and H1-AHP of the 2nd generation (non-sedative) – modern.
How allergic reactions develop in the human body
I generation H1-AHPs include: diphenhydramine, clemastine, dimethindene, chloropyramine, mebhydroline, hifenadine, hydroxyzine, etc. I generation antihistamines are able to cross the blood-brain barrier and, therefore, bind to H1 receptors in the brain.
Sedative-hypnotic effect when taken is observed in 40-80% of patients. Its absence in individual patients does not exclude the objective negative effect of these drugs on cognitive functions (memory, learning ability, driving a car).
Due to the non-selectivity of action and influence on other receptors (M-cholinergic receptors, serotonin, alpha-adrenergic receptors) and ion channels, 1st generation antihistamines can cause dry mucous membranes, trembling, sinus tachycardia, urinary retention, constipation, hypotensive effect.
In addition, high doses of some antihistamines are toxic, especially to children. Due to the competitive blocking of histamine H1 receptors, the therapeutic effect of first generation antihistamines is quickly reversible, which requires the use of drugs of this group several times a day.
It should also be taken into account that the effectiveness of drugs in the course of treatment decreases: in the 1st week. application, there is a therapeutic effect, on the 2nd week. the phase of addiction begins, and on the 3rd week. – side effect phase. Therefore, H1-1st generation AGP should not be used for more than 14 days.
Despite the fact that H1-HPA of the first generation can cause the side effects listed above, they are still widely used in clinical practice today. H1-AGP of the 1st generation has one advantage – the availability of injectable forms that are indispensable in the provision of emergency care, premedication before certain types of diagnostic examinations and surgical interventions.
Currently, a large clinical experience has been accumulated in the use of first-generation antihistamines, but clinical studies that meet the requirements of evidence-based medicine have not been conducted.
The presence of pronounced side effects of the first generation H1-AHP contributed to the development of the second generation AHD. The main differences of H1-AGP II generation are high selectivity and specificity of action, lack of sedation and drug tolerance (tachyphylaxis).
Modern AHDs have been synthesized over the past 20 years by modifying known compounds. Therefore, according to the chemical structure, they belong to the same classes as the first generation AGP. II generation antihistamines have a high affinity for H1-histamine receptors, are characterized by a rapid onset of action, the duration of the effect is up to 24 hours
They have the ability to selectively act on H1 receptors, being antagonists, they transfer them to an inactive state without violating their physiological properties. These drugs do not cross the blood-brain barrier, so they cause little or no drowsiness.
AHPs have some significant additional antiallergic effects:
they stabilize the mast cell membrane, reduce the expression of adhesion molecules (ICAM-1), inhibit eosinophil-induced release of interleukin (IL)-8, granulocyte-macrophage colony-stimulating factor and soluble ICAM-1 from epithelial cells.
Therefore, they are more effective than first-generation antihistamines in the long-term therapy of allergic diseases, in the genesis of which mediators of the late phase of allergic inflammation play a significant role. The evidence base for the efficacy and safety of second-generation antihistamines is very solid [13].
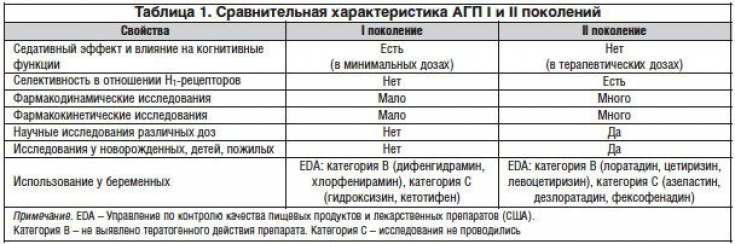
Comparative characteristics of AGP I and II generations are presented in Table 1.
II generation antigens are a heterogeneous group, primarily due to the peculiarities of their metabolism. Among them, 2 subgroups are distinguished:
- metabolizable drugs that have a therapeutic effect only after transformation in the liver under the influence of the CYP 3A4 isoenzyme of the cytochrome P450 system with the formation of active compounds. These include: loratadine, ebastine, terfenadine, astemizole;
- active metabolites – drugs that enter the body in the form of an active substance (cetirizine, levocetirizine, desloratadine, fexofenadine). They have a more favorable safety profile, the effect of these drugs is more predictable and does not depend on the activity of enzymes of the cytochrome P450 system, so their use is more preferable.
The advantages of active metabolites, the intake of which is not accompanied by an additional burden on the liver, are obvious: the speed and predictability of the development of the effect, the possibility of co-administration with various drugs and foods that are metabolized with the participation of cytochrome P450.
Due to the variety of AHD, it is quite difficult to make the right choice between one or another drug. The efficacy and safety of the new generation of antihistamines have been demonstrated in numerous randomized, double-blind clinical trials. In clinical practice, the doctor should be guided by modern data of evidence-based medicine, according to which II-generation H1-AHP, in particular desloratadine, are first-line drugs for the treatment of a wide range of diseases.
Desloratadine is the active metabolite of – a drug that enters the body in the form of an active substance, which ensures its higher safety profile.
It was synthesized in 1998 and registered in Russia in 2001. Desloratadine has the ability to suppress the acute phase of an allergic response by blocking H1 receptors. Experimental studies have shown that desloratadine has the highest affinity for H1 histamine receptors and slow dissociation from their association.
Desloratadine binds noncompetitively to H1 receptors and has been clinically shown to be 52, 57, 194, and 153 times more potent than cetirizine, ebastine, fexofenadine, and loratadine, respectively. The drug is rapidly absorbed after oral administration and is characterized by a high rate of reaching the maximum plasma concentration and a rapid onset of action (after 1.25-3 hours).
The pharmacokinetics of desloratadine is linear and dose proportional. The half-life of the drug is 21-24 hours, which allows you to prescribe it 1 r./day. Eating does not affect the rate and degree of absorption of the drug. It was found that the pharmacokinetics and bioavailability of desloratadine were similar when taking the drug on an empty stomach or after a standardized meal in healthy people (the maximum concentration on an empty stomach and after a meal was 3.3 and 3.53 ng / ml, respectively, p = 0.17). Therefore, the drug can be taken both after meals and on an empty stomach, which indicates the convenience of its use. Metabolism and excretion of the drug do not depend on the age and sex of the patient.
Animal, in vitro and in vivo studies have shown that desloratadine, by inhibiting a number of inflammatory mediators, has additional anti-allergic and anti-inflammatory effects that are not associated with the blockade of H1-histamine receptors.
In physiological concentration, the drug effectively inhibits the production of histamine-dependent pro-inflammatory cytokines – IL-6 and IL-8, which are known to stimulate the secretion of pro-inflammatory mediators such as tumor necrosis factor-α when released from endothelial cells, basophils and mast cells. Desloratadine has an effect on the activation and survival of eosinophils.
Eosinophils, being key effector cells in an allergic reaction, produce cytokines, chemokines, leukotrienes, and neuromodulators. In addition, desloratadine, through inverse agonism, reduces the expression of nuclear factor κB (NF-κB), known as an inducer of RANTES, the main attractant for eosinophils, monocytes and T-lymphocytes, promoting the activation of eosinophils and the release of histamine from basophils.
Desloratadine inhibits the activity of NF-κB, which stimulates the release of pro-inflammatory mediators from basophils and mast cells, more than other antihistamines. In this effect, the drug is superior to cetirizine, loratadine and fexofenadine. Recent studies have shown that desloratadine may also inhibit mast cell degranulation and subsequent histamine release. In addition, desloratadine inhibits histamine-induced P-selectin expression.
Desloratadine is characterized by a high level of safety in its use. It does not cause negative changes in the cardiovascular system and other organs, does not have a hypnotic effect and does not affect cognitive functions. The drug can be used by patients with pathology of the hepatobiliary system and kidney diseases, it is approved for use in children from 1 year old.
Desloratadine stands out among the representatives of second-generation antihypertensive drugs for more than 10 years of successful experience in wide medical use and a large evidence base. The efficacy and safety of desloratadine in the treatment of patients with chronic idiopathic urticaria (CUI) has been proven by numerous randomized, placebo-controlled clinical trials (Fig. 1).
J. Ring, R. Hein, A. Gauger conducted a double-blind, placebo-controlled, multicenter study that included 190 patients with moderate to severe CCI. During exacerbation of the disease, the 1st group of patients was prescribed desloratadine 5 mg/day, the 2nd group – placebo (control). The duration of treatment reached 6 weeks. The primary end point was the mean change in pruritus index during the first 7 days of treatment compared to baseline. It was found that during the 1st week. in patients treated with desloratadine, the pruritus index decreased by 56%, and in the control group – by 22%, there was also a more rapid regression of skin rashes than in the control group. It was found that in patients from the 1st group at the end of the 1st week. treatment, the degree of sleep disturbance when using the drug decreased by 53%, and in patients from the 2nd group – only 18%. After 6 weeks against the background of taking the drug, the itching index decreased by 74%, and against the background of taking placebo – by 48.7%. By the end of the study, in patients taking desloratadine, the degree of sleep disturbance decreased by almost 80%. Both patients and doctors highly appreciated the overall positive dynamics of CJK symptoms and response to desloratadine treatment. The frequency of adverse events was comparable in 2 groups, no serious adverse reactions were registered [25].
the degree of sleep disturbance decreased by almost 80%. Both patients and doctors highly appreciated the overall positive dynamics of CJK symptoms and response to desloratadine treatment. The frequency of adverse events was comparable in 2 groups, no serious adverse reactions were registered [25].the degree of sleep disturbance decreased by almost 80%. Both patients and doctors highly appreciated the overall positive dynamics of CJK symptoms and response to desloratadine treatment. The frequency of adverse events was comparable in 2 groups, no serious adverse reactions were registered [25].
In a later study, 137 patients with moderate to severe CUC were randomized into 2 groups. Patients from the 1st group were prescribed desloratadine 5 mg/day, from the 2nd – placebo for 6 weeks. By the end of the study, the pruritus index in the desloratadine group decreased by 1.43, and in the placebo group – by 0.86 (p=0.004). After 6 weeks the number of patients with a complete, significant or moderate response to treatment was higher in the group of patients treated with desloratadine compared with patients treated with placebo (68.8 and 36.8%, respectively). No serious adverse events were reported, and the incidence of any adverse reactions was 11.1% in the placebo group and 6.2% in the desloratadine group.
In another study, desloratadine was shown to reduce the severity of the main clinical symptoms of CIU, primarily itching, by 50-70%. The effect of the drug lasted up to 24 hours. Reduction in symptoms of itching at the end of the dosing interval was noted in 45% (versus 4% taking placebo) and 69% after 6 weeks. reception. There was also a significant decrease in the size and number of blisters on the background of desloratadine therapy with its long-term use. Patients reported an 80% improvement in sleep. Assessment of the quality of life in patients with CCI while taking the drug for 7 days showed a decrease in scores on the scale of the Dermatology Life Quality Index (DLQI) questionnaire from 13.4 to 9.1. In 60% of patients during the indicated period, the DLQI index decreased by an average of 2 points. By the end of the study, the proportion of such patients reached 77% (p < 0.0001).
The efficacy and tolerability of desloratadine in patients with allergic diseases was also studied in 4 large clinical trials in Germany in 2001-2002. The total number of patients of both sexes over the age of 12 was 77,800. Symptoms of allergic diseases were assessed before and after treatment. As a result of treatment with desloratadine, the vast majority of patients experienced relief of symptoms, which indicated a pronounced clinical effect. Moreover, a rapid onset of action while taking desloratadine was noted by 67% of patients and 63% of physicians. In addition to the relief of the main symptoms of allergic diseases, the majority of patients improved their general condition in the form of normalization of sleep and increased daily activity.
In a clinical study involving 12,050 patients, the high therapeutic efficacy of desloratadine in CCI was confirmed. At the same time, it was noted that the antihistamine and antiallergic activity of the drug is not accompanied by the effect of sedation and does not affect cognitive and psychomotor functions (attention concentration, memory, learning ability). This allows long-term use of the drug in outpatient practice.
In Russia, a number of studies have been conducted on the effectiveness of the use of desloratadine in the treatment of various dermatosis, accompanied by subjective sensations in the form of itching. Yu.V. Sergeev et al. the effectiveness of desloratadine was evaluated in the treatment of 26 children aged 5 to 15 years suffering from atopic dermatitis (Fig. 2). The duration of the disease ranged from 4 to 12 years. Children under 12 years of age received the drug in syrup, 2.5 mg in 5 ml 2 times a day for 14 days. In children over 12 years of age, a tablet form of 5 mg / day was used.
At the time of treatment, other drugs, including glucocorticosteroids, were canceled. It was shown that 14 days after the appointment of desloratadine, itching, weeping, and the size of foci decreased significantly, the SCORAD index decreased (by 5 or more times), as well as the level of total immunoglobulin (Ig) class E and the frequency of detection of IgE antibodies to food and household allergens. In general, in almost 90% of children, 2-week therapy with desloratadine contributed to the reduction of itching or its complete regression. No adverse reactions were noted.
The study of the efficacy and safety of desloratadine was conducted by N.V. Kungurov et al. in patients with allergic dermatoses. The authors showed that in atopic dermatitis and eczema, along with histamine, pruritogenic effects were maintained by an increased content in the body of other biologically active substances, such as serotonin, bradykinin, kallikrein, proteases, prostaglandins, leukotrienes, and eicosanoids. The ongoing therapy with desloratadine 5 mg/day in combination with external non-hormonal therapy gave a positive effect in the form of a reduction in itching or its complete regression in 90% of patients. No side effects have been reported during treatment with desloratadine [31].
I.M. Korsunskaya et al. evaluated the antipruritic activity of desloratadine in patients with photodermatitis, solar eczema, urticaria, eczema and atopic dermatitis. All patients with an acute course of the process were prescribed desloratadine at a dose of 5 mg/day for 2 weeks; in a chronic course, the duration of therapy was 3 weeks. In all cases, the treatment was effective, a positive effect in the form of itching relief was achieved within 3-7 days of therapy. All patients with photodermatitis, solar eczema, urticaria experienced clinical recovery after the end of therapy, patients with eczema and atopic dermatitis showed a significant improvement in the form of the absence of itching and a decrease in erythema.
According to international and national consensus documents, the use of non-sedating II generation antihistamines is recommended as the first line of drug therapy for AR and hay fever. Desloratadine meets all ARIA/EAACI criteria and is recommended as first line treatment for AR.
A recent study at the Institute of Immunology examined the efficacy and safety (tolerability) of desloratadine in patients with seasonal AR. The study included 30 patients (12 men and 18 women) with AR in the period of exacerbation, who received desloratadine 5 mg/day for 28 days. Patients daily assessed the dynamics of AR symptoms, as well as the need for topical decongestants before the start of the course of treatment and during the period of therapy. All 30 patients treated with the drug noted a decrease in the severity of AR symptoms (nasal discharge, mucus dripping down the back of the throat, sneezing and nasal congestion), as well as eye symptoms (itchy eyes, tearing and redness of the eyes).
Improvement was noted by the end of the 1st week. admission, and this trend persisted for 4 weeks. observations. By the end of the course of treatment, 73% of patients noted complete remission and significant improvement. Studies have shown that desloratadine is highly effective in reducing nasal and ocular symptoms in patients with seasonal AR and conjunctivitis.
It has a good safety profile, leads to an improvement in the patient's quality of life and can be recommended as monotherapy in patients with AR with a mild course of the disease and in complex therapy in patients with AR with moderate and severe course.
In AR, nasal decongestants for topical use in the form of drops or spray and aerosol are often included in the complex therapy. One of the actively used drugs in clinical practice is Evkazolin. The main component of the drug – xylometazoline, has a vasoconstrictive and anti-edematous effect, as a result of which nasal breathing is restored. Due to the composition of eucalyptus oil, the drug has anti-inflammatory and antimicrobial effects.
The drug has an α-adrenomimetic effect, reduces hyperemia, exudation, facilitates nasal breathing. Thanks to the eucalyptus oil included in its composition, the drug eliminates the dryness of the nasal mucosa and has an anti-inflammatory and antiseptic effect.
It acts mainly locally, when used in therapeutic doses, it is absorbed through the mucous membranes in small quantities. The action begins a few minutes after application, lasts for 8-10 hours. Indications for the use of the drug: acute rhinitis and rhinosinusitis, hay fever; otitis media (to reduce swelling of the mucous membrane of the nasopharynx); as well as preparing the patient for diagnostic and therapeutic procedures in the nasal passages.
In Russia, Evkazolin is available in the form of Evkazolin Aqua spray. The drug is prescribed for adults and children over the age of 12, 1 injection of 2-3 rubles / day in each nasal passage. Duration of use of the drug – 5–7 days.
Thus, desloratadine can be used in acute and chronic skin diseases both in the form of monotherapy and as part of complex therapy. Desloratadine has the highest affinity for the H1 receptor, slowly dissociates, has the properties of both a neutral antagonist and an inverse agonist, and has the longest half-life compared to other H1-AGP representatives of the second generation. It does not have a sedative effect and does not cause other adverse reactions associated with exposure to the central nervous system. The drug is effective in the treatment of AR, pruritic dermatosis, in particular, such as atopic dermatitis, allergic contact dermatitis, eczema, urticaria, lichen planus, mastocytosis, as well as idiopathic pruritus.
A patient's adherence to a particular therapy and to a particular drug depends on the efficacy, safety level, ease of use, and cost of the drug. Since most allergic diseases are chronic and the patient pays for the treatment out of his own funds, the affordability of the drug is of great importance.
Elisey – a histamine H1 receptor blocker (long-acting) is the primary active metabolite of loratadine. Inhibits the release of histamine and leukotriene C4 from mast cells. Prevents the development and facilitates the course of allergic reactions.
It has antiallergic, antipruritic and antiexudative action. Reduces the permeability of capillaries, prevents the development of tissue edema, relieves spasm of smooth muscles. It practically does not have a sedative effect and, when taken in a dose of 7.5 mg, does not affect the speed of psychomotor reactions. In comparative studies of desloratadine and loratadine, there were no qualitative or quantitative differences in the toxicity of 2 drugs at comparable doses (taking into account the concentration of desloratadine).
Elyseum is used to relieve symptoms associated with the following conditions: AR (sneezing, nasal discharge, nasal itching and congestion, itchy and red eyes, watery eyes, itchy palate); urticaria (itching, rash). The drug is taken orally at the same time of day.
For adults and children over 12 years of age, the drug is prescribed at a dose of 5 mg (1 tablet) 1 r./day, regardless of food intake. The duration of treatment depends on the severity and course of the disease. Treatment of intermittent AR (presence of symptoms for less than 4 days in 1 week or less than 4 weeks) should be carried out taking into account anamnestic and clinical data: stop after the disappearance of symptoms and resume after their reappearance. With persistent AR (presence of symptoms for more than 4 days in 1 week or more than 4 weeks), treatment should be continued during the entire period of exposure to the allergen.
Thus, the use of antihistamines effectively eliminates the symptoms of allergic reactions, improving the quality of life of patients.
Allergens in autumn: the main causes of the disease






Add a comment