Galderma Laboratories, LP announced that the Food and Drug Administration (FDA) has approved Mirvaso®; (brimonidine) - gel for the topical treatment of erythema (redness) in rosacea in adults (18 years of age and older). Applied just once a day, Mirvaso works quickly to reduce the redness of rosacea with effects that last up to 12 hours. According to Galderma, Mirvaso will be available in pharmacies this month.
"Facial flushing is the most common symptom of rosacea, but until now there has been no drug that would solve this patient problem," said Mark Jackson, MD, professor of clinical medicine at the University of Louisville, dermatologist and principal investigator of Mirvaso. "The FDA approval of Mirvaso Gel marks a turning point in the treatment of erythema: we can now offer patients who deal with the daily frustration of rosacea redness an effective therapy."
Mirvaso's approval was based on data from more than 550 patients enrolled in two Phase 3 clinical trials. The results of both studies showed that the subjects who used Mirvaso showed a significant improvement in skin condition. In addition, a long-term study was also conducted with 276 patients who used Mirvaso for up to 12 months.
Mirvaso is a safe and well-tolerated gel-based 0.33% brimonidine that constricts dilated facial blood vessels to reduce facial redness associated with rosacea.
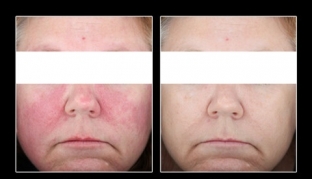
Mirvaso gel: before and after photo (3 hours after application).







Add a comment