Mitochondrial labile iron (LI) is a major contributing factor in skin fibroblast susceptibility to UVA-induced oxidative damage, resulting in death cells as a result of ATP depletion. Mitochondrial iron overload is a key feature of the neurodegenerative disease Friedreich's ataxia — FRDA.
In an experiment, cultured primary skin fibroblasts from patients with Friedreich's ataxia have been shown to be 4-10 times more sensitive to UVA than their healthy counterparts.
The cells of FRDA patients have been shown to contain higher levels of mitochondrial labile iron (increased by up to 6 times compared to controls) and higher generation of mitochondrial reactive oxygen species after UVA irradiation (up to 2 times on average). In the article estet-portal.com you can read the results of the study in detail.
Relationship between iron and UV sensitivity
Iron is an indispensable element of life as it is involved in several important cellular functions. However, iron can also be potentially cytotoxic when present in the form of redox chelated labile iron (LI), which can act as a catalyst in the formation of harmful substances. Therefore, LI levels are usually tightly regulated in cells.
Most intracellular labile iron is found in subcellular compartments with mitochondria.
Due to their function in respiration, mitochondria are also the main source of oxygen free radicals in cells, hence the presence of high-intensity LI in mitochondria makes these organelles particularly susceptible to oxidative stress.
Follow us on Instagram
Iron is a vital element for cellular processes such as mitochondrial energy production. It is known that mitochondria are the main target of UVA radiation damage in skin fibroblasts, leading to ATP depletion and subsequent cell death.
Friedreich's Ataxia — ultraviolet hypersensitivity test field
Given the central role of mitochondria in cells, it is not surprising that the dysregulation of mitochondrial iron metabolism in Friedreich's ataxia has profound implications for cell integrity and function.
Friedreich's Ataxia — is a disease caused by insufficient levels of frataxin (FXN), a mitochondrial protein that plays a key role as an iron chaperone in the synthesis of heme and iron sulfur clusters (ISCs) and in antioxidant defense.
The consequence of a pathological or genetically determined decrease in FXN levels in cells and tissues is an overload of mitochondria with iron.
Photoaging is reversible: ways to prevent premature aging
Iron chelator therapy for hypersensitivity to ultraviolet light
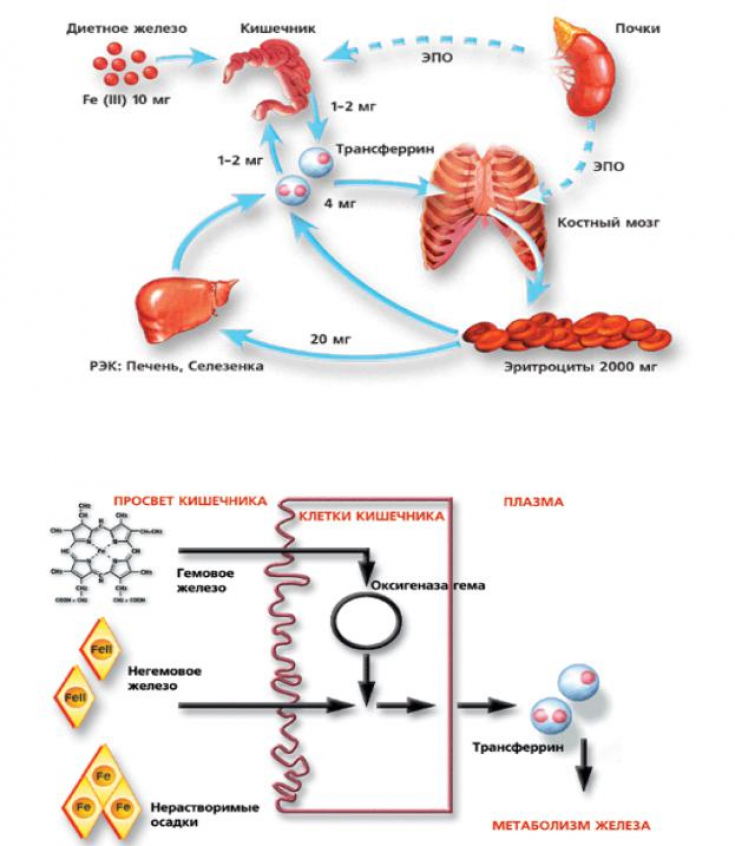
Pretreatment of human fibroblasts prior to UVA irradiation with mitochondria-targeting iron chelator deferiprone (DFP) has been shown in an experiment to inhibit LI-catalyzed free radical production, cell death, and halve both mitochondrial membrane damage and ATP depletion in human fibroblasts FRDA.
Cytotoxicity caused by iron and oxygen free radicals can be partially abolished if cells are pretreated with iron chelators.
There is clearly potential for the use of iron chelators to restore mitochondrial function and cell/tissue integrity in FRDA.
However, all chelators used systemically to date, including DFP, have disadvantages of lack of target specificity with respect to the site of injury (i.e., mitochondria) and associated risks, including disruption of normal homeostasis and depletion of the patient's iron levels resulting in anemia, hence there is a clear need to develop iron chelators with improved properties.
Iron deficiency anemia: an insidious killer of beauty and health
One approach to reduce/prevent unwanted effects and increase the effectiveness of the chelator is to target the chelating unit to the mitochondria by linking it to a mitochondrial-specific "SS-peptide". Such peptides, based on alternating hydrophobic and basic amino acid side chains, easily penetrate cell plasma membranes and selectively accumulate in mitochondria.
The authors have demonstrated the successful use of an iron chelator targeting hexadentate mitochondria to photoprotect cultured skin fibroblasts from solar UVA (320-400 nm), hence induced mitochondrial damage and subsequent cell death.
Practical value: determination of UV sensitivity and its correction
Mitochondrial labile iron levels can be used as a predictor of skin photosensitivity.
Although they lie deep in the skin, fibroblasts are a major target for long-wavelength UVA photodamage of the skin, as up to 50% of this ultraviolet range penetrates the dermis.
UVA directly affects the structure of the dermis through an increase in the activity of matrix metalloproteinases, which leads to the remodeling of the extracellular matrix due to the degradation of collagens and elastin, which are the hallmarks of photoaged skin. The results highlight the possibility that sensitization of other skin cells may occur in other diseases characterized by mitochondrial iron overload.
In this regard, the topical application of mitochondrial-targeting iron chelators such as cpd 2 in sunscreen can be considered a valuable approach for skin photoprotection in patients with FRDA and other iron storage pathologies.
Thank you for staying with estet-portal.com. Read other interesting articles in the "Cosmetology" section. You might be interested in Sunscreens: 5 Criteria for Safety and Effectiveness







Add a comment