Spasticity of the upper extremities in children is a serious problem and can negatively affect the development and quality of life of the child. To treat this condition, today, physiotherapy is most often used, however, it is not able to quickly improve the child's condition, and botulinum toxin – maybe.
June 21, 2019, BOTOX® was approved by the FDA for the treatment of upper limb spasticity not only in adults but also in children.
On estet-portal.com read more about the use of botulinum toxin for the treatment of this condition in pediatric patients.
Main causes of upper limb spasticity
Injury to the brain and spine can lead to spasticity, which is often seen as increased muscle resistance and tone in the upper and lower extremities.
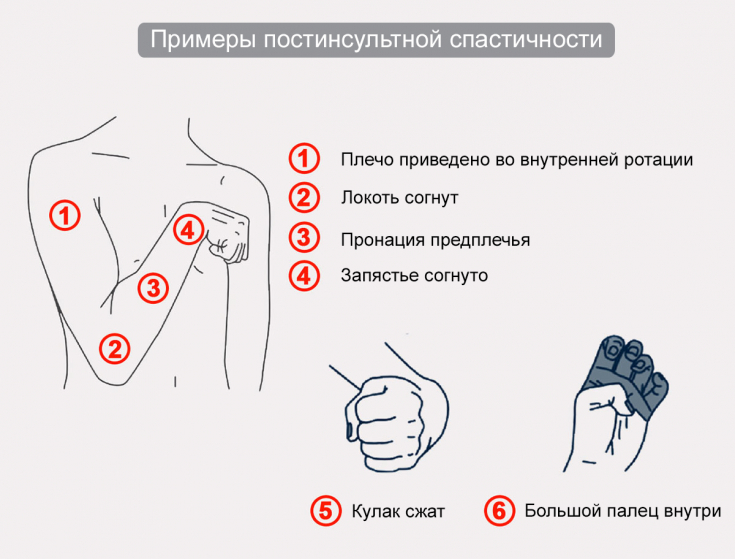
Spasticity of the upper extremities can interfere with the normal movement of the arms, up to the complete disruption of their work.
Common causes of spasticity in children include:
• cerebral palsy;
• traumatic brain injury;
• multiple sclerosis;
• spinal cord injury and stroke.
These disorders impair the ability to move normally and maintain balance and posture.
Botulinum toxin recovery: important aspects of the use of the drug
Botulinum toxin against spasticity of the upper limbs

BOTOX preparation® – it is the first neurotoxin approved for the treatment of children aged 2 to 17 years with upper limb spasticity.
BOTOX® has a well-established safety and efficacy profile and may be an important treatment option for successfully controlling upper limb spasticity in children and adolescents.
It is important to understand that drug treatment cannot replace physical therapy or other rehabilitation prescribed by a physician.
The approval of BOTOX® for the treatment of upper limb spasticity is based on data from two studies evaluating the safety and efficacy of BOTOX® in more than 200 pediatric patients with upper limb spasticity.
These trials included a 12-week double-blind study and a one-year open-label extension study.
FDA Approved – another win for Allergan
The FDA approval is special to everyone at Allergan because the company can now provide children with a real help in treating spastic conditions with BOTOX®.
Company "Allergan" also looks forward to the FDA's decision on the treatment of spasticity in children.
FDA approval means a lot to Allergan as the company is always committed to working with doctors, healthcare professionals and patients to provide innovative and meaningful therapies that help people around the world improve their quality and life expectancy.
Read the most interesting articles in Telegram!
Required dose for correcting upper limb spasticity with botulinum toxin
The approved recommended dose per treatment session is 3 units per kilogram to 6 units per kilogram divided between the affected muscles of the upper extremity. The total dose for pediatric patients should not exceed 8 units per kilogram of body weight or 300 units, whichever is less, at intervals of 3 months.
Dose of the product is not equal or comparable to another botulinum toxin product.
Original drugs of the company "Allergan" You can only purchase from an authorized distributor.
Possible complications of botulinum therapy and their prevention







Add a comment