A prospective, randomized, blind study of the effect of botulinum toxin preparations was conducted in 30 patients with symmetrical moderate-severe forehead lines using a 4-point scale for assessing facial wrinkles.
The objectives of the study were to evaluate the efficacy and durability of unreduced incobotulinumtoxinA with unreduced onabotulinumtoxinA.
Find out in our article on estet-portal.com the latest research on the action of botulinum toxin , characteristics of the types of neurotoxin, their differences and subsequent techniques for improving the structure and formula of the drug.
- Areas of application of botulinum toxin
- The main components of botulinum toxin
- Clinical studies of botulinum toxin
- results action of botulinum toxins
Areas of application of botulinum toxin
Botulinum toxin (BoNT) is used to treat various neurological disorders and is widely used in aesthetic medicine as a main weapon in the fight against age-related changes and provides significant aesthetic improvement to the skin. There are currently seven different serotypes of neurotoxin in use. There are serotypes of botulinum toxin: types A, B, C, D, E, F and G.1.
Follow us on Instagram !
Each of these neurotoxins has a unique molecular structure and function, and each is produced from a different strain of the bacteria Clostridium Botulinum. Currently, three formulations of botulinum toxin A (BoNT-A) are commonly used: Botox, incobotulinumtoxinA, and onabotulinumtoxinA.
The drugs OnabotulinumtoxinA and incobotulinumtoxinA are two components of botulinum toxin A (BoNT-A) commonly used in aesthetic medicine. Their main differences depend on whether complexing proteins are included in the active neurotoxin or not.
While OnabotulinumtoxinA has complexing proteins, IncobotulinumtoxinA does not. However, it is unclear whether these differences affect their effectiveness, durability, or immunogenicity. using.
The main components of botulinum toxin
Each of these drugs has its own unique benefits; however, it is unclear whether their structural and functional differences are clinically significant. Factors that distinguish each neurotoxin include dose potency or equivalence, onset of action, duration of action, local diffusion, side effect profile, and differences in immunogenicity. The main difference between these different neurotoxin formulations is the presence or absence of complexing proteins.
10 interesting facts about botulinum toxin: everything you want to know
Manufacturers typically produce botulinum toxin as a 150-900 kDa protein that includes both the primary active component (150 kDa polypeptide chain) and complexing proteins. The 150 kDa protein is a neurotoxin and has low toxic activity; however, after cleavage into its 50 kDa (light chain) and 100 kDa (heavy chain) components, the toxin activity increases. Complexing proteins consist of hemagglutinin and smaller non-hemagglutinin proteins.
Complexing proteins are sometimes called accessory proteins, protective proteins, or neurotoxin proteins. They are important for protecting toxins in their natural environment (pH range 5-7), but dissociate at physiological pH 6-8.
OnabotulinumtoxinA contains complex-forming proteins, while incobotulinumtoxinA does not. The amount of botulinum toxin product, along with complexing proteins and residual proteins, determines the foreign protein load on the human body. The human immune system can recognize any part of this protein as part of a neurotoxin, which is recognized as a foreign substance and causes an immune response, especially after injection.
Clinical studies of botulinum toxin
Several studies, mostly in the clinical literature, have suggested that higher total protein content may increase the risk of antibody formation. As a consequence, botulinum toxin A products began to be produced with a corresponding reduction in total protein content. The current incobotulin A formula contains only 5 ng of complexing protein per 100 units (U).
Rejuvenation of the male face with botulinum toxin type A preparations
Clinically, however, it is unclear whether these molecular differences are significant and have an impact on antigenicity and efficacy. Due to the large number of non-randomized, non-blind, industry-sponsored studies, clinicians have difficulty determining whether a particular botulinum toxin product is more beneficial than another in terms of efficacy and safety.
A prospective, randomized, blinded clinical trial was conducted. The study protocol was approved by the committee of The Esthetic Clinics Institute.
Botulinum toxin: the hottest news of botulinum therapy
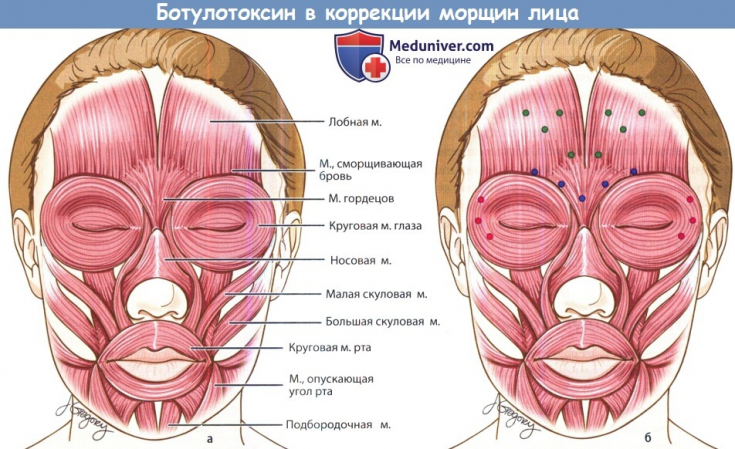
Thirty patients with symmetrical, moderately severe forehead wrinkles with maximum frowning took part in the study. The two groups were matched by age and gender to avoid any confounding results or biases. To assess the wrinkle line, the international Carruthers scale.
Results of botulinum toxin action
The Clinical Improvement Scale was used to analyze the study results and patient outcomes were assessed over a 24-week period. The analysis found that forehead lines reappeared in patients treated with onabotulinumtoxinA after 8.3 weeks, compared with 10.1 weeks in patients treated with incobotulinumtoxinA.
Bright head or botulinum therapy against migraine
While improvement in both forehead lines was observed for both toxins after 8 weeks.
The efficacy and improvements of onabotulinumtoxinA compared to incobotulinumtoxinA decreased, indicating that incobotulinumtoxinA was more effective in long-term wrinkle improvement.
Botulinum toxin: why the poison has no analogues among drugs







Add a comment