Stem cells regenerate tissues under conditions of homeostasis and stress. Receiving signals from their microenvironment, they smoothly move from a state of rest to activation. Immune cells act as helpers for stem cells throughout the body.
Here we explore the context-specific interactions that stem cells have with resident cells and recruited immune cells (cells of the innate immune system such as macrophages and dendritic cells, as well as adaptive immune T cells).
Read in the estet-portal.com article about this fascinating cross-control that holds great promise for new treatments for inflammatory diseases and regenerative medicine.
Role of stem cells in self-renewal mechanisms
Self-renewing tissue stem cells are associated with a balancing act of proliferation and differentiation that maintains a balance between hyperplasia and atrophy. The rate of cell replacement during homeostasis depends on tissues and background.
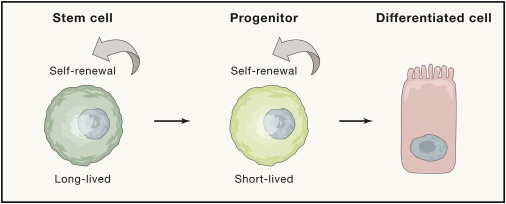
The renewal process is continuous in the blood, epidermis and intestines; limited in brain and muscles; and episodic in the hair follicle and lactating mammary gland. However, when tissues are damaged, stem cells can be mobilized into action on an emergency basis. The highest immune activity is observed in the epithelial tissues of the skin, lungs and intestines.
Watch the most interesting videos on our
Immune effectors quickly come from the vessels, penetrate the stressed tissue to cleanse it of penetrating pathogens, help restore homeostasis.
Skin immunity: nuances that were not known before Two tissue-resistant immune cell types - macrophages and regulatory T cells (Tregs) - have emerged as potent regulators of stem cells under normal physiological conditions. Macrophages, classically known as professional phagocytes due to their ability to engulf dead and dying cells, were among the first identified immune cell types capable of modulating stem cells. In the bone marrow, macrophages regulate the maintenance of hematopoietic stem cells (HSC).
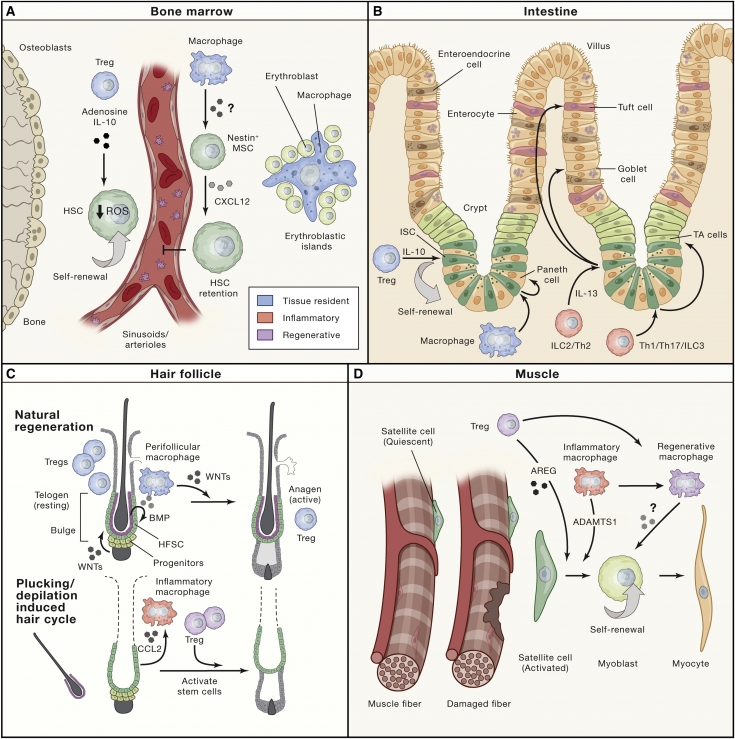
Activation of this natural cyclical regenerative process requires changes in the stem cell niche and includes both dampening of quiescence signals (BMP) and increased activation of stem cells and regenerative signals (WNT).
While the origin of resting signals has been attributed to terminal differentiated stem cell progeny (BMPs) that line the "inner niche" as well as long range signals from the dermis; resonant macrophages have been identified as stem cell activators.
During the progression from dormancy to the regenerative phases, macrophages gather around the follicle. These perifollicular macrophages die and release WNT7b and WNT10a to activate hair follicle stem cells (HFSC).
Coordinated work – key to successful stem cell activation
Thus, the physiological process of transition from telogen to anagen involves many molecular and cellular mechanisms, which are coordinated due to the balanced interaction of stem cells and cells of the immune system.
In this regard, there are three obvious ways in which these interactions can be used for therapeutic benefit:1. strengthening of immune mediators to promote regeneration,
2. correction of immune abnormalities underlying tissue degeneration,
3. interrupting this dialogue to limit pathologies in autoimmune.
Thank you for staying with estet-portal.com. Read other interesting articles in the "Trichology" section. You may be interested in
Anagen Alopecia: Causes and Treatment
Cell







Add a comment